[Courtesy of SK Bioscience]
SEOUL -- SK Bioscience, a cooperation partner for South Korea's state project to develop COVID-19 vaccines, will immediately embark on the first phase of clinical trials to evaluate the safety and immunogenicity of its vaccine candidate called NBP2001. The first test will be conducted on healthy adults at a state-run hospital.
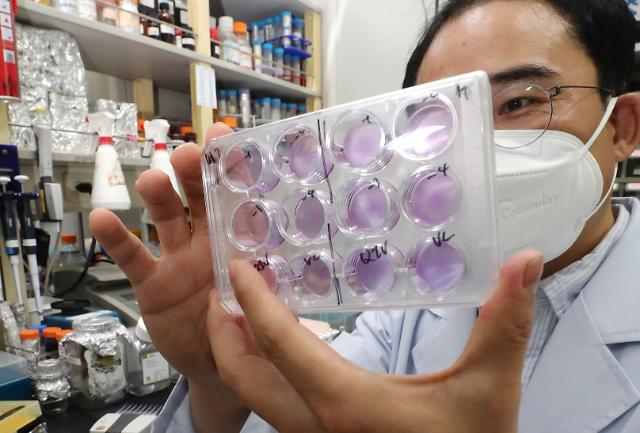
The Ministry of Food and Drug Safety said that SK Bioscience has been allowed to carry out clinical trials of NBP2001, a recombinant vaccine manufactured from the virus' surface antigen protein through genetic combination. The surface antigen protein stimulates immune cells to induce immune responses. SK Bioscience claimed to have proved the effectiveness of NBP2001 through pre-clinical experiments on animals.
"Our goal is to make a COVID-19 vaccine with proven safety and effectiveness, even if it's a little later than global vaccines," SK Bioscience CEO Ahn Jae-yong, showing confidence over the development of a safe vaccine through the protein culture and refining platform of an antigen produced with gene recombination technology.
Based on the excellent level of non-clinical results verified on several occasions, SK Bioscience vowed to carry out quick clinical trials, saying that mass production is possible as soon as development is completed. The company has already succeeded in developing candidate substances for a cervical cancer vaccine through the same synthetic antigen method and completed second-stage clinical trials.
The Korea National Institute of Health, a state research body, has selected SK Bioscience as a cooperation partner for its project to develop preventive vaccines and medicine. In addition to NBP2001, SK Bioscience is conducting the pre-clinical test of another vaccine candidate GBP510. The company aims to start clinical trials within this year.
SK Bioscience has led a government drive to acquire vaccines through various channels. In July, the company signed a deal with AstraZeneca PLC, a Swedish-British multinational company, to produce undiluted solutions and finished products.
AstraZeneca became the third pharmaceutical company to announce positive results from late-stage trials of a coronavirus vaccine, saying that its candidate, developed by Oxford University, is up to 90 percent effective. Pfizer and its German partner BioNTech and Moderna have each reported vaccines that were 95 percent effective in clinical trials.
In August, SK Bioscience secured a deal to produce the antigen of a vaccine candidate being developed by Novavax, an American vaccine development company, that would be supplied in South Korea and globally if it proves to be safe and effective through clinical trials. Novavax is undergoing clinical trials for its vaccine candidate, NVX-CoV2373.
South Korea has unveiled a roadmap for the treatment of COVID-19 to develop plasma treatment by the year's end as well as antibody treatment and vaccines next year. Currently, clinical trials of 19 treatments and three vaccines are underway. The other two vaccine candidates were DNA vaccines that induce immune responses by injecting surface antigen protein genes.