[Courtesy of KIMS]
A clinic-level PCR-based virus detection method using molecular diagnostic techniques offers high sensitivity and specificity. However, the method requires a large space and expensive equipment. Samples are collected at COVID-19 screening centers before being tested at designated labs. It takes at least four hours to get results.
RPA-based tests are an efficient isothermal method that requires a shorter test process and less equipment. Because of relative simplicity, RPA is ideal for onsite tests in resource-limited environments such as makeshift screening centers.
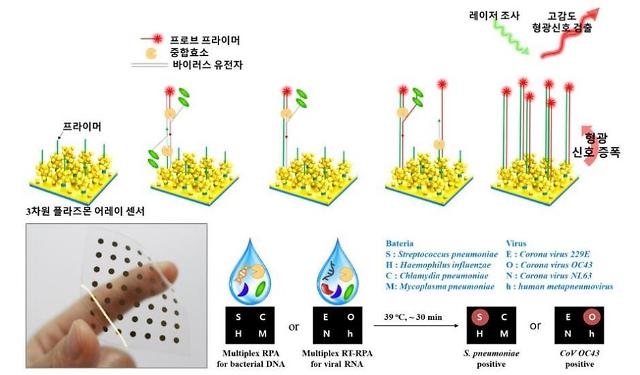
The Korea Institute of Materials Science (KIMS) said in a statement on April 6 that researchers from the institute and Samsung Medical Center have developed the world's first rapid and sensitive multiplex molecular diagnosis method for respiratory pathogens using a plasmonic isothermal RPA array chip. The RPA chip can be used outside labs.
"This approach enables rapid, sensitive and high-multiplex molecular detection and can be used in the realization of a simplified and miniaturized platform for onsite multiplex molecular diagnostics," researchers said in a paper published on the website of Biosensors and Bioelectrics, an international scientific journal.
The RPA chip composed of silver nanoparticles on dense silver nanopillars successfully detected bacterial DNA within 30 minutes and viral DNA within 40 minutes, the research team said, adding the chip's sensitivity was far higher than liquid-based PCR techniques, and no cross-reactivity was witnessed.
KIMS and Samsung Medical Center will cooperate with a domestic clinical test equipment maker to accelerate the commercialization of the RPA array chip while conducting a clinical demonstration on COVID-19 patients to receive state approval.




